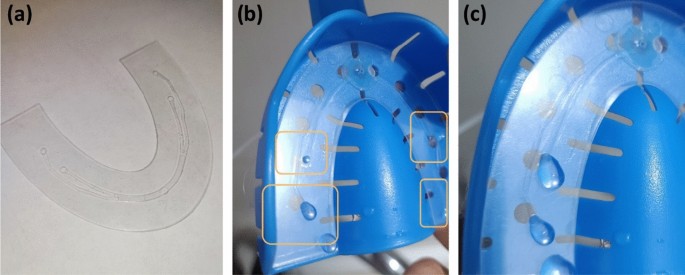
A custom-made intra-oral portable micro-electronic (IOPM) theranostic device was effectively designed and fabricated, as illustrated in Fig. 5. The central element of this IOPM theranostic device was the dental impression tray, typically employed in crafting a dental anatomical model.
The artificial tooth model was constructed using acrylic resin. Initially, the tooth model was positioned within a dental impression tray to create an impression using a transparent vinyl polysiloxane material35. This material became sturdy after setting, making it easy to cut and drill injection openings that corresponded to the outlets of the previously described microfluidic chip. Figure 5a displays the developed portable prototype of an electronic device for an intra-oral drug delivery system, featuring embedded sensors for monitoring the oral cavity’s temperature and humidity. The molded dental model held within the dental impression tray is presented in Fig. 5b.
The complete IOPM prototype consisted of the following parts: a small transparent fluid chamber and a small lithium-polymer (LiPo) battery for powering the device. The battery was placed out the side making the final device wearable inside the mouth. When the battery was turned on, the original output of 3.7–4 V was low. Thus, a voltage booster was used to increase the voltage up to 5 V. The microcontroller atmega328p (Fig. 5a) was used to drive the system. One switch was placed to prevent the battery from powering the system for safety reasons. The bottom side of the device will have direct contact with the tooth and saliva. Therefore, a sensor SHT35 was placed in this location to measure the exact temperature and humidity in the oral cavity.
Figure 6 illustrates the components of a fabricated microfluidic chip (Fig. 6a) and testing results that depict liquid dispensing from each microfluidic outlet (Fig. 6b,c).
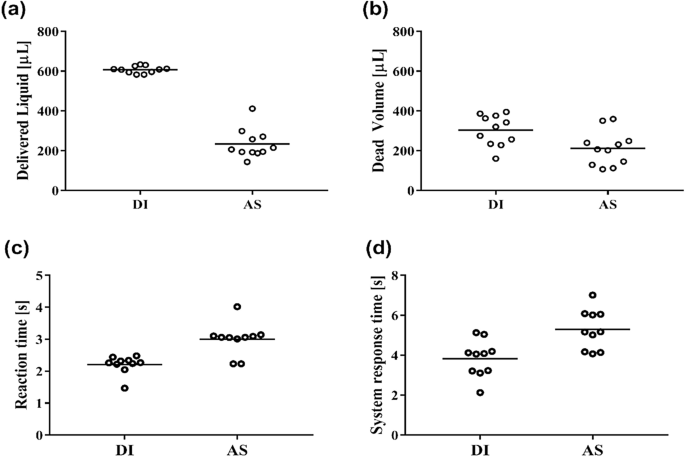
The IOPM theranostic device platform has been tested successfully for DI and AS fluids delivery efficiency and temperature-dependent reaction time for liquid dispensing. Table 1 shows the descriptive data analysis for the entire liquid dispensing sample tested. Mean values with standard deviations for the volume of the Delivered Liquid and the Dead Volume of the whole system. The normality of distribution was tested using the Shapiro–Wilk test. Since the data showed the normal distribution, parametric methods and Student t-tests were employed for the data analysis.
The experimental results demonstrated a significant difference when using DI water and AS for the dead volume, as shown in Table 1 and Fig. 7a. The difference is reflected in the amount of Delivered Liquid, which differs almost three times. We expected such results considering these two liquids’ different viscosities and surface tension. In a Newtonian fluid, the flow rate in cylindrical tubing decreases as viscosity increases according to Poiseuille’s law36. Hence, for the same parameters (micropump settings, duration of discharge), we would get a reduced release of liquid using AS, which was also demonstrable by measurement.
Table 2 and corresponding to Fig. 7a,b show the values of the comparative analysis of two volumes (the Delivered Liquid and the Dead Volume) of both examined fluids (Student’s t-test), where Delivered Liquid-DI and Delivered Liquid-AS, as well as Dead Volume-DI and Dead Volume-AS were compared. Results showed that the obtained values are statistically significant for tested liquids and all tested parameters (p < 0.05, Student’s t-test). Dead volume represents fluidic retained inside the microfluidic chip and tubing.
The recorded measurement represents the volume of fluid released on dentures in the practical application of this device is presented in Fig. 7a. The Dead Volume (remaining fluid in the chamber) was also measured and is shown in Fig. 7b.
In order to determine-temperature dependent system reaction time, we measured the reaction time from the beginning of the temperature change until the start of the system (when the first droplet exits the system). The temperature reaction time is shown in Fig. 7c. In the case of DI water, the system reaction time was statistically significantly shorter (p < 0.05, Mann–Whitney test).
Furthermore, we measured the system response time–time needed to start the system from an empty system (system full of air) until the start of the system (system full of liquid)—when the first droplet shows at the microfluidic outlets (Fig. 6b). These system response times are depicted in Fig. 7d. Similarly, for DI water, the system response time was statistically significantly shorter (p < 0.05, Mann–Whitney test).
The studies analyzing intraoral temperature have yielded several significant findings. There are reports in the literature that, for healthy adults, the optimal range for intraoral temperature falls between 36.8 and 37.0 °C. At the same time, intraoral temperature fluctuations were investigated with a focus on inter-racial variation and association with outside temperature37. The temperatures measured at the incisor and premolar had median values of 34.9 °C and 35.6 °C, respectively, and ranged from 5.6 to 58.5 °C and 7.9 to 54 °C, respectively. There was just a weak correlation between intraoral temperatures and ambient temperature. This study also discovered that the temperature distributions between the Asian and Caucasian groups were significantly different. During the 24-h period, temperatures at the incisor site were in the range of 33–37 °C for around 79% of the time, and 92% of the time at the premolar site. But, when it comes to equally important intraoral underlying structures, research has indicated that on average, the temperature of the alveolar bone is about 5 degrees Celsius lower than that of the mucosa covering it38. Temperature variations have been observed in many physiological and pathological conditions. For example, it has been reported for gingival lesions, generally being warmer than the gingival crest, while chronic lesions tend to exhibit lower temperatures39. But depending on the environmental influences, some reports go in line with the finding that the physiological temperature range of 20–60 °C40. When it comes to pathological conditions, it has been reported that acute lesions were found to be warmer than the gingival crest, while chronic lesions generally exhibited cooler temperatures41. These findings emphasise the importance of maintaining an appropriate range of intraoral temperature for overall oral health and comfort and relevance of our developed device to efficiently monitor the temperature and the temperature changes within the above mentioned clinically relevant temperature range, and detect the temperature changes.
There is a limited amount of information available in the literature regarding temperature sensors designed for use inside the mouth. They are made with the intention of precisely measuring the temperature of the mouth cavity. In one method, temperature measuring electrodes are provided in a flexible portion of the catheter shaft and a catheter is equipped with temperature sensors. Temperature sensors are installed into conductive columnar bodies and connected to the electrodes used to detect temperature through these electrodes42.
Wearable intraoral sensors have been created with a wide range of characteristics and capabilities. These sensors utilize small strain gauge sensors to measure the forces exerted by the tongue and lips, and they employ an integrated circuit (IC) sensor interface to manage multiple sensors concurrently. In addition, these sensors incorporate a low-energy transmission module to process signals and wirelessly transmit data using the Bluetooth® Low Energy protocol. The design of these sensors places importance on correcting output errors caused by initial strain in real-time, as well as achieving compact dimensions and wireless data transmission, thereby eliminating the necessity for external cables. Digital intraoral sensor designs differ across various models, showing variations in cable arrangements, connectivity interfaces, the inclusion of radiation shields for back-scattering, plate thickness, and the active sensor area. Nonetheless, the industry lacks standardization, and manufacturers often do not readily disclose specific details about the physical design43. Table 3 summarises the key findings and comparison of the temperature measurement in oral cavity by researchers.
The design of a wearable intraoral diagnostic platform is a promising field in biomedical engineering. It involves integrating a powerful system-on-chip processor, a smart power management unit, and multi-function peripherals into a miniature module. Some wearable intraoral diagnostics use a spatiotemporal nano-/microstructural arrangement, consisting of hierarchically stacked geometrical configuration based free-standing films formed of porous, fibrous, and naturally helical cellulose networks. These films have functionalized hybrid spin-sensitive graphene-ink printed sensing layers that are scalable, binder-free, and printed without a binder46. The HSGC’s time–space-resolved architecture produces a mass spectrogram, allowing for the separation, elution, and detection of distinct molecules within mixtures. This architecture offers real-time and wide-spectrum molecular analysis for various biomedical applications, such as wearable spectrometry and quick on-site biopsy decision-making for surgical oncology48.
Humidity sensors have various applications in dentistry and clinical medicine. In healthcare settings, they regulate humidity for optimal conditions. Organic substances, like molecular rectifiers, offer precise humidity data43. Metal–organic frameworks and their derivatives also exhibit great potential as materials for humidity sensing, primarily due to their remarkable porosity and stability49. Moreover, the development of printed capacitive humidity sensors, featuring stacked parallel-plate electrodes, has yielded sensors that possess high capacitance and sensitivity, all while maintaining an economical cost. Furthermore, substrates coated with moisture-sensitive substances can be employed to gauge moisture levels by detecting alterations in light output6. In healthcare, humidity sensors in mobile apps monitor patients’ conditions. These apps save data in the cloud for analysis by medical professionals or caregivers50.
Intraoral humidity can be measured via various methods, such as using an intraoral moisture meter or measuring breath humidity. Dentine adhesion studies have also investigated the influence of relative air humidity and temperature on moisture in the oral cavity, considering factors such as the use of a rubber dam and nose/mouth breathing51.
Exaclear GC, a widely used clear vinyl polysiloxane, is extensively employed in the technique of Injection Moulding for a variety of purposes. Its application is commonly observed in aesthetic and wear cases, as well as in temporary crown and bridge work. Furthermore, Exaclear GC is highly appropriate for the layering of composite materials, particularly when light-curing is necessary from the palatal side. Moreover, its efficacy extends to bite registration and the facilitation of the transfer of brackets and fibers from the model to the oral cavity52,53,54. Vinyl polysiloxane compounds have a significant impact on the field of dentistry due to their extensive range of applications, exceptional biocompatibility, and adherence to high safety standards. These compounds are frequently utilized in the creation of dental impressions, which serve as an accurate replication of the oral tissues for various restorative procedures such as crowns, bridges, and dentures. By offering superior detail reproduction and dimensional stability, VPS materials guarantee the precise fitting of prosthetic restorations. Additionally, these materials have proven to be safe for intraoral usage as they possess low toxicity, limited allergy potential, and excellent tissue compatibility. The hydrophobic nature of vinyl polysiloxane materials also plays a crucial role in effectively managing moisture during the impression-taking process. Consequently, due to their remarkable biocompatibility and safety features, these materials have become a favored option in contemporary dentistry, facilitating successful and dependable outcomes for both patients and dental professionals55,56. Previous studies have made attempts to incorporate sensors into vinyl polysiloxane (VPS) devices for various applications. For instance, researchers have explored the adaptation of dental appliances to measure intraoral pressure during functional activities57. This innovative approach enables the assessment of pressure changes within the oral cavity, providing valuable insights into occlusal forces and bite dynamics. In another investigation, the performance of a temperature sensor embedded into a mouthguard was examined13. This research aimed to evaluate the accuracy and reliability of temperature measurements during oral activities. Additionally, the potential of an oral-based bioguard to estimate heart rate using photoplethysmography has been investigated58. By integrating sensors into VPS devices, these studies have demonstrated the versatility and potential of vinyl polysiloxane materials in creating functional and biocompatible dental appliances capable of monitoring physiological parameters.
Currently, intraoral sensors and drug delivery devices use an oral appliance or wearable patch to be placed in the oral cavity for an extended amount of time. For example, France et al.24 introduced a system for ultrasound-based drug delivery in the oral cavity to treat inflammatory diseases using delivered therapeutics to mucosal surfaces. However, that system does not contain any sensors. Additionally, the same group of researchers developed a microneedles patch to deliver macromolecules and applied it to the buccal area25. On the other hand, Shi et al.26 reported a dental patch that can wirelessly, using NFC, observe the oral microenvironment (e.g., pH value) and deliver drugs if it is necessary. The miniature device reported in Ref.26 was intended to be mounted on the tooth and may cause discomfort or interfere with the visual and functional appearance of the subject. Our proposed IOPM theranostic device is based on a conventional dental tray which is regularly used in clinical practice, and we have enhanced this tray with embedded sensors and precise fluid-delivering functionalities.
One major advantage of the proposed system is that it is only required to be used when necessary by the user or clinician rather than being worn continuously like previously reported drug delivery systems. This allows for greater flexibility, comfort, and convenience for patients and dentists, as they can use the tray as needed for their treatment or diagnosis and then remove it when finished (similar to taking dental impressions, which is a very common procedure). This could lead to greater patient adherence and satisfaction with the treatment process, as wearable intraoral appliances can cause discomfort. In addition, the microfluidics and device design could be customised for each patient using casting mould for a personalised treatment process targeting certain areas of the denture. An instance of clinical usage is that the smart dental tray could deliver artificial saliva to the denture depending on the oral environment (temperature and humidity) and will be helpful for patients suffering from xerostomia.
One limitation of the system is that the proposed device is not intended for continuous monitoring and delivery; it is designed for on-demand or intermittent use. Currently, this proof-of-concept system has been demonstrated using readily available sensors. In the future, this electronic microfluidic platform can be enhanced by incorporating additional sensors, such as pH sensors and electrochemical biomarker sensors, enabling precise medication delivery to specific locations for personalized diagnosis, treatment, and improved drug administration. Furthermore, the system in its entirety can be further miniaturized and customized to better fit within a mouthguard. This device offers real-time feedback and data for both patients and dentists, potentially enhancing treatment outcomes.
https://www.nature.com/articles/s41598-023-48379-9